Jonathan Heddle, PhD is Head of Bionanoscience and Biochemistry Laboratory at the Malopolska Centre of Biotechnology, Jagiellonian University in Kraków, Poland. IIOL talks to Dr. Heddle about his research project creating ultra-stable gold-coordinated protein cages and their possible future applications.
How did your research experience in Poland begin?
Dr. Heddle: While I was working in Japan, I learnt about an opportunity in Kraków, with the potential for good funding and support. These are crucial for research to succeed but not always easy to find. It turns out that there are good opportunities in Poland for scientists with vision.
What is your research?
Dr. Heddle: A large part of our research is in the field of bionanoscience. For us, this means trying to design and build functional biological nanomachines and nanoscale structures that can be programmed to carry out desired functions, particularly related to treatment of disease.
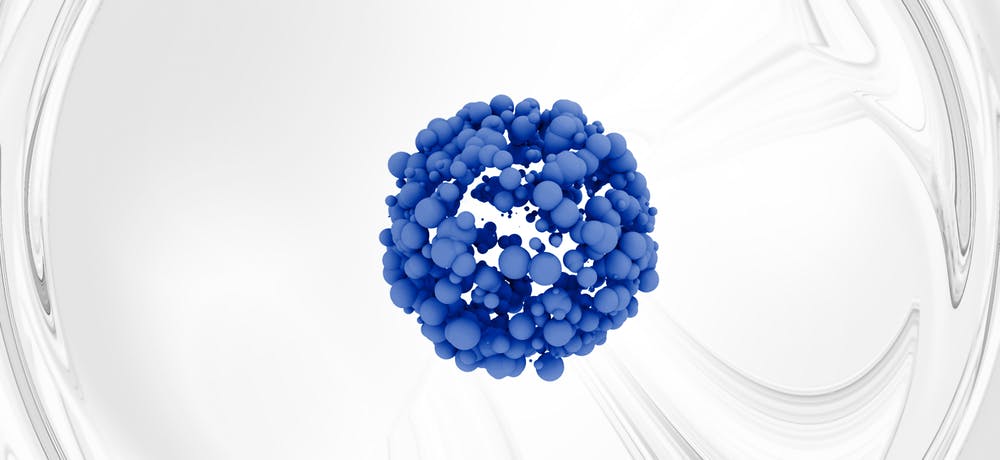
In our case for example, we are trying to develop new DNA origami structures that may even be useful for constructing artificial cells. But our most advanced work right now is a novel protein cage that we made. It is just 22 nanometers in diameter (a nanometer being one billionth of a meter or something like one hundred thousand times smaller than the diameter of a human hair). These cages are extremely stable (they survive boiling temperatures) but easily break down in conditions similar to what is found in our own cells. If the hollow interior is filled with a drug then this has obvious potential for drug delivery to our cells and we are interested in now finding ways to target it to cells affected by age-related diseases.
How advanced is your research?
Dr. Heddle: Our research is a very new area and is a platform from which other things may grow, so that question is difficult to answer. Computer science can be a good analogy: If you asked people two decades ago if advanced computing “intelligence” would be useful in medicine, it may have been difficult to say. Now, we use artificial intelligence to analyze medical data and diagnose patients. That’s a good example of developing something fundamental, a platform: it is the core technology that allows further developments, sometimes years later. Bio-nanoscience is still at this very early stage, we are building its core capabilities.
The structures researchers are able to make now are relatively simple. But using DNA, and a technique called DNA origami, it has already been proven possible to build box-like “nanorobots” that can be programmed to open in response to particular molecular signals (including disease signals). The potential for putting a toxic drug in the box and only releasing it in the vicinity of a diseased cell is obvious.
Ultimately, the most sophisticated machines in nature are proteins (just think of the amazing things enzymes can do) and while it is harder to engineer programmable protein structures, we are getting there, with protein cages being a promising field at the moment. These are artificial protein “balls” with hollow interiors. The hope is that we may be able to engineer such cages so that they carry drugs into cells and then only release the drugs when certain conditions are met. In other words the ultimate, targeted drug delivery system. There are many age-related diseases where this could be useful. The most obvious would be in numerous cancers where drugs, if not exquisitely targeted, end up killing healthy cells, often with severe side effects.
There are a lot of age-related diseases that could be treated with smart drug delivery systems.
How does your research apply to aging?
Dr. Heddle: Diseases of aging are typically multifaceted – many things go wrong at the same time as we age – and often, unlike infectious diseases, diseases of aging involve the malfunctioning of our own cells and proteins. This can make them difficult to treat as anything that targets the body’s own cells could end up affecting healthy ones as well as diseased ones. Unfortunately, most drugs in use are still pretty dumb – small molecules that we ingest or inject that are distributed across virtually the whole body – potentially affecting healthy as well as diseased cells. In principle, by using bionanoscience we can build more complex “machines” with capabilities more like we see in nature – structures with the ability to move, make decisions etc. Imagine what kind of sophisticated capabilities such new drugs could have or how such structures could be used to more smartly deliver existing drugs so as to increase effectiveness.
There are a lot of age-related diseases that could be treated with smart drug delivery systems. For instance, age is the biggest risk factor for cancer. Many drugs have been developed to treat various cancers and now researchers want to figure out how to target the right cells with them, to make the treatment more effective and efficient.
In the future, research into nano and miscroscale systems, including enzymes, could lead to creating programmable artificial systems that can detect anomalies, digest molecules, find and repair damaged cells, and even do DNA repair. All of these are age-related issues.
What will the next stage of your research be?
Dr. Heddle: We have a basic functioning protein cage model. We can trigger its assembly and disassembly. We can trigger the conditions, with the view for potential drug delivery. But there’s still a long road ahead before actual medical application.
Our next step is to improve and refine it, increasing complexity: Perhaps we can build capsules within capsules, improve their programmability, attach molecular “locks” or even multiple locks on these cages so that they will open if the right, very specific conditions are met, e.g. such as when it encounters a receptor on the cell that marks it as cancer. We have almost all the required techniques and technologies to do this in vitro, but we need further refinement and they need to be biocompatible in order to have a chance of working.
Ultimately, I think sophistication and programmability of protein machines will grow significantly. From drug delivery to actual correction of the root cause (i.e. bionano structures able to replace or repair damaged structures in cells). It could even be possible that like some components of our own immune system, we could have artificial bionano structures in our bodies constantly, monitoring for errors to arrive when we age and then instantly correcting them. Though we are some way off that prospect at the moment.
How soon do you think these nanomachines could be used in medicine?
Dr. Heddle: The answer could be never – if for instance, we discover that these kind of structures trigger a huge immune response. That is what trials, first in vitro, then further down the line in clinical trials, will determine. If things go very well, we may see something on the decade timescale but prediction is always tricky.

